emission line
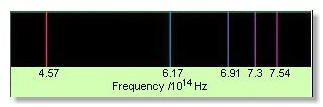
Figure 1. The Balmer spectrum of hydrogen.
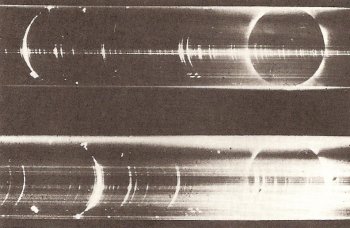
Figure 2. Flash spectrum of the Sun during a solar eclipse. Image: Harvard College Observatory.
An emission line is a bright line in the spectrum of a luminous object caused by the emission of light at a particular wavelength. Emission lines may appear on their own, as in the spectrum of a nebula energized by radiation from a nearby hot star, or they may be superimposed on an absorption spectrum, as happens when a star is surrounded by hot gas.
An emission spectrum is a spectrum that consists predominantly or solely of emission lines (Figure 1).
Notable emission lines include the D lines of sodium in the yellow part of the spectrum.
Flash spectrum
A flash spectrum is an emission line spectrum of the solar chromosphere, obtained by placing an objective prism in front of the telescopic lens the instant before or after totality in a solar eclipse (Figure 2).


