lead-acid battery
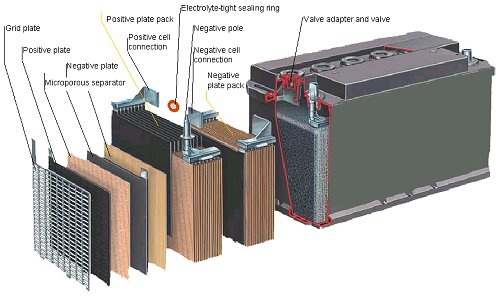
A lead-acid battery is an electrochemical battery that uses lead and lead oxide for electrodes and sulfuric acid for the electrolyte. Lead-acid batteries are the most commonly used in photovoltaic (PV) and other alternative energy systems because their initial cost is lower and because they are readily available nearly everywhere in the world. There are many different sizes and designs of lead-acid batteries, but the most important designation is whether they are deep cycle batteries or shallow cycle batteries.
Shallow cycle batteries, like the type used as starting batteries in automobiles, are designed to supply a large amount of current for a short time and stand mild overcharge without losing electrolyte. Unfortunately, they cannot tolerate being deeply discharged. If they are repeatedly discharged more than 20 percent, their life will be very short. These batteries are not a good choice for a PV system.
Deep cycle batteries are designed to be repeatedly discharged by as much as 80 percent of their capacity so they are a good choice for power systems. Even though they are designed to withstand deep cycling, these batteries will have a longer life if the cycles are shallower. All lead-acid batteries will fail prematurely if they are not recharged completely after each cycle. Letting a lead-acid battery stay in a discharged condition for many days at a time will cause sulfation of the positive plate and a permanent loss of capacity.
Sealed deep-cycle lead-acid batteries are maintenance free. They never need watering or an equalization charge. They cannot freeze or spill, so they can be mounted in any position. Sealed batteries require very accurate regulation to prevent overcharge and over discharge. Either of these conditions will drastically shorten their lives. Sealed batteries are well-suited for remote, unattended power systems.
