alpha particle

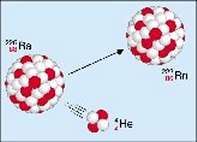
The decay of radium-226 into radon-222 with the emission of one alpha particle. Image credit: European Nuclear Society.
An alpha particle is a kind of particle emitted spontaneously during the type of radioactive decay known as alpha decay. An alpha particle is identical with the nucleus of a helium atom (4He), consisting of two protons and two neutrons. The rest mass of the alpha particle is 6.64424 × 10-27 kilograms, or 3.7273 × 109 eV. Alpha particles have a low penetrating power and a short range (a few cm in air). The most energetic of them (up to 7.5 MeV) will generally fail to penetrate the dead layers of cells covering the skin and can be easily stopped by a sheet of paper. Alpha particles play an important role in nuclear fusion processes within stars.
The spontaneous emission of an alpha particle occurs in elements of mass number greater than about 150, such as uranium, thorium, and plutonium. For example, 238-U → 234-Th + 4He, in which the uranium nucleus changes into a different element, thorium, because two protons have been emitted from the original nucleus.
During alpha decay the atomic number is reduced by two units and the mass number by four units. For example, alpha decay generates Rn-222 with the atomic number 86 and the mass number 222 from Ra-226 with the atomic number 88 and the mass number 226.
An alpha particle emitted by a uranium nucleus has an initial speed of about 15 million m/s (about 5% of the speed of light), which is fast but not as fast as a lighter beta particle. However, as a result of its high mass and relatively slow speed, combined with its positive charge, an alpha particle can easily remove electrons from – i.e. cause ionization of – the atoms in a substance, and its energy of motion is transferred to the medium. As it transfers energy it slows down. The rate of transfer depends on the medium. Alpha particles can penetrate up to 75 millimeters in air but much less in solid matter. They are said to have a high linear energy transfer (LET) and it is the rapid loss of energy in a small range which makes them such a radiation hazard if ingested.
Compare with beta particles and gamma rays.


